SYNTHESIS OF STARCH AND SUCROSE CSIR TOPIC PLANT PHYSIOLOGY
SYNTHESIS OF STARCH AND SUCROSE
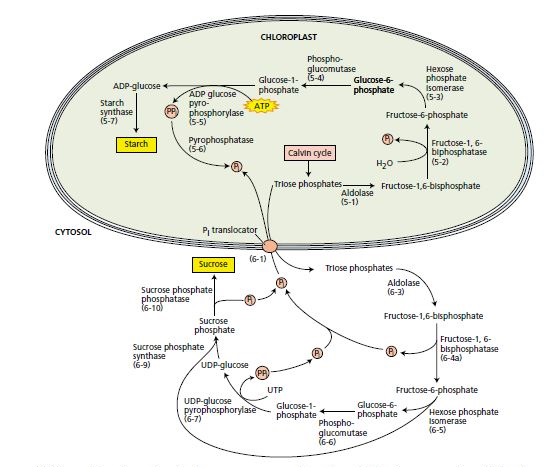
In most species, sucrose is the principal form of carbohydrate translocated throughout the plant by the phloem. Starch is an insoluble stable carbohydrate reserve that is present in almost all plants. Both starch and sucrose are synthesized from the triose phosphate that is generated by the Calvin cycle. The pathways for the synthesis of starch and sucrose are shown in below figure.
Starch Is Synthesized in the Chloroplast
The prominent starch deposits, as well as enzyme localization studies about the chloroplast , leave no doubt that the chloroplast is the site of starch synthesis in leaves. Starch is synthesized from triose phosphate via fructose-1,6-bisphosphate. The glucose-1-phosphate intermediate is converted to ADP-glucose via ADP-glucose pyrophosphorylase in a reaction that requires ATP and generates pyrophosphate (PPi, or H2P2O72–). As in many biosynthetic reactions, the pyrophosphate is hydrolyzed via a specific inorganic pyrophosphatase to two orthophosphate (Pi) molecules.. Finally, the glucose moiety of ADP-glucose is transferred to the non reducing end (carbon 4) of the terminal glucose of a growing starch chain , thus completing the reaction sequence.
Sucrose Is Synthesized in the Cytosol
The site of sucrose synthesis has been studied by cell fractionation, in which the organelles are isolated and separated from one another. Enzyme analyses have shown that sucrose is synthesized in the cytosol from triose phosphates by a pathway similar to that of starch—that is, by way of fructose-1,6-bisphosphate and glucose-1-phosphate. In sucrose synthesis, the glucose-1-phosphate is converted to UDP-glucose via a specific UDP glucose pyrophosphorylase that is analogous to the ADP glucose pyrophosphorylase of chloroplasts. At this stage, two consecutive reactions complete the synthesis of sucrose. First, sucrose-6- phosphate synthase catalyzes the reaction of UDP-glucose with fructose-6 phosphate to yield sucrose-6 phosphate and UDP. Second, the sucrose-6- phosphate phosphatase (phosphohydrolase) cleaves the phosphate from sucrose-6-phosphate, yielding sucrose . The latter reaction, which is essentially irreversible, pulls the former in the direction of sucrose synthesis. As in starch synthesis, the pyrophosphate formed in the reaction catalyzed by UDP-glucose pyrophosphorylase is hydrolyzed, but not immediately as in the chloroplasts. Because of the absence of an inorganic pyrophosphatase, the pyrophosphate can be used by other enzymes, in transphosphorylation reactions. One example is fructose-6-phosphate phosphotransferase, an enzyme that catalyzes a reaction like the one catalyzed by phosphofructokinase except that pyrophosphate replaces ATP as the phosphoryl donor. A comparison of the reactions in reveals that the conversion of triose phosphates to glucose-1-phosphate in the pathways leading to the synthesis of starch and sucrose have several steps in common. However, these pathways utilize isozymes (different forms of enzymes catalyzing the same reaction) that are unique to the chloroplast or cytosol. The isozymes show markedly different properties. For example, the chloroplastic fructose-1,6-bisphosphatase is regulated by the thioredoxin system but not by fructose- 2,6-bisphosphate and AMP. Conversely, the cytosolic form of the enzyme is regulated by fructose-2,6-bisphosphate (see the next section), is sensitive to AMP especially in the presence of fructose-2,6-bisphosphate, and is unaffected by thioredoxin. Aside from the cytosolic fructose-1,6 bisphosphatase, sucrose synthesis is regulated at the level of sucrose phosphate synthase, an allosteric enzyme that is activated by glucose-6-phosphate and inhibited by orthophosphate. The enzyme is inactivated in the dark by phosphorylation of a specific serine residue via a protein kinase and activated in the light by dephosphorylation via a protein phosphatase. Glucose-6-phosphate inhibits the kinase, and Pi inhibits the phosphatase. The recent purification and cloning of sucrose-6-phosphate phosphatase from rice leaves is providing new information on the molecular and functional properties of this enzyme. These studies indicate that sucrose-6-phosphate synthase and sucrose-6-phosphatase exist as a supramolecular complex showing an enzymatic activity that is higher than that of the isolated constituent enzymes. This noncovalent interaction of the two enzymes involved in the last two steps of sucrose synthesis points to a novel regulatory feature of carbohydrate metabolism in plants.
The Syntheses of Sucrose and Starch Are Competing Reactions
The relative concentrations of orthophosphate and triose phosphate are major factors that control whether photosynthetically fixed carbon is partitioned as starch in the chloroplast or as sucrose in the cytosol. The two compartments communicate with one another via the phosphate/ triose phosphate translocator, also called the phosphate translocator , a strict stoichiometric antiporter. The phosphate translocator catalyzes the movement of orthophosphate and triose phosphate in opposite directions between chloroplast and cytosol. A low concentration of orthophosphate in the cytosol limits the export of triose phosphate from the chloroplast through the translocator, thereby promoting the synthesis of starch. Conversely, an abundance of orthophosphate in the cytosol inhibits starch synthesis within the chloroplast and promotes the export of triose phosphate into the cytosol, where it is converted to sucrose. Orthophosphate and triose phosphate control the activity of several regulatory enzymes in the sucrose and starch biosynthetic pathways. The chloroplast enzyme ADP-glucosepyrophosphorylase is the key enzyme that regulates the synthesis of starch from glucose- 1-phosphate. This enzyme is stimulated by 3-phosphoglycerate and inhibited by orthophosphate. A high concentration ratio of 3-phosphoglycerate to orthophosphate is typically found in illuminated chloroplasts that are actively synthesizing starch. Reciprocal conditions prevail in the dark. Fructose-2,6-bisphosphate is a key control molecule that allows increased synthesis of sucrose in the light and decreased synthesis in the dark. It is found in the cytosol in minute concentrations, and it exerts a regulatory effect on the cytosolic interconversion of fructose-1,6-bisphosphate and fructose-6-phosphate :
Increased cytosolic fructose-2,6-bisphosphate is associated with decreased rates of sucrose synthesis because fructose- 2,6-bisphosphate is a powerful inhibitor of cytosolic fructose- 1,6-bisphosphatase and an activator of the pyrophosphate-dependent (PPi-linked) phosphofructokinase (reaction 4b). But what, in turn, controls the cytosolic concentration of fructose-2,6-bisphosphate? Fructose-2,6-bisphosphate is synthesized from fructose- 6-phosphate by a special fructose-6-phosphate 2-kinase Recent evidence suggests that, as in animal cells, both plant activities reside on a single polypeptide chain. The kinase and phosphatase activities are controlled by orthophosphate and triose phosphate. Orthophosphate stimulates fructose-6-phosphate 2-kinase and inhibits fructose- 2,6-bisphosphatase; triose phosphate inhibits the 2- kinase. Consequently, a low cytosolic ratio of triose phosphate to orthophosphate promotes the formation of fructose-2,6-bisphosphate, which in turn inhibits the hydrolysis of cytosolic fructose-1,6-bisphosphate and slows the rate of sucrose synthesis. A high cytosolic ratio of triose phosphate to orthophosphate has the opposite effect. Light regulates the concentration of these activators and inhibitors through the reactions associated with photosynthesis and thereby controls the concentration of fructose- 2,6-bisphosphate in the cytosol. The glycolytic enzyme phosphofructokinase also functions in the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate, but in plants it is not appreciably affected by fructose- 2,6-bisphosphate. The activity of phosphofructokinase in plants appears to be regulated by the relative concentrations of ATP, ADP, and AMP. The remarkable plasticity of plants was once again illustrated by recent gene deletion experiments with transformed tobacco plants. This experiment shows that the transformed plants can grow without a functional pyrophosphate-dependent fructose-6-phosphate kinase enzyme. In this case the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate is apparently catalyzed exclusively by phosphofructokinase .
Thank You
Team Crest
Aarif Meo
9050321626
Please Follow Us by a Click









Comments
Post a Comment
Thanks for Comment....